Researchers Develop Method for Identifying Radiation-Resistant Tumors
New research at the University of Arkansas has found a way to identify radiation-resistant cancer cells, a breakthrough in the treatment of lung cancer that uses imaging to evaluate the response to treatment and match treatment to specific tumor cells.
Until now, there has been no way to determine immediately after beginning therapy if a tumor is resistant to radiation therapy or not. The findings were published this month in Scientific Reports, a Nature journal.
“Our results demonstrate that the use of autofluorescence imaging of cell metabolism can identify treatment-resistant cancer cells,” said Narasimhan Rajaram, assistant professor of biomedical engineering at the University of Arkansas and lead researcher on the project. “More importantly, we think that this technique provides a sound method to evaluate tumor response to treatment and match tumors to the right therapy.”
Using an innovative imaging system, a team of researchers from the University of Arkansas and the University of Arkansas for Medical Sciences has identifed differences between the metabolic response of radiation-resistant and radiation-sensitive lung cancer cells following therapy.
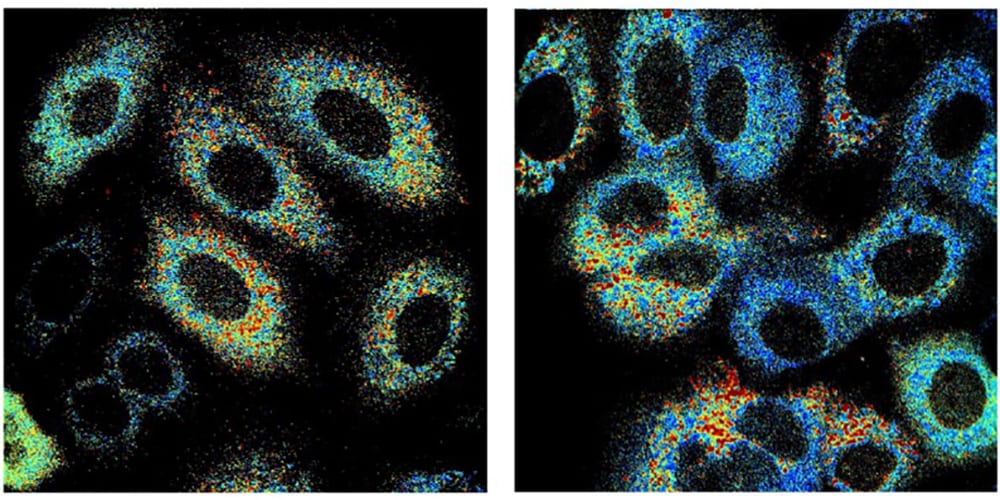
The researchers used autofluorescence imaging to observe changes in two molecules – nicotinamide adenine dinucleotide (NADH), and flavin adenine dinucleotide (FAD) – that both naturally absorb and emit light. These molecules play a critical role in the major metabolic pathways within cells that are responsible for respiration and energy production. By measuring the relative contributions of NADH and FAD to these processes, researchers can evaluate biochemical details related to cell metabolism.

Narasimhan Rajaram
Previous research by this team compared radiation-resistant cancer cells to raditation-sensitive cells and found that radiation-resistant cells have higher levels of hypoxia-inducible factor, which regulates the amount of oxygen in the cellular environment and protects the cancer cells from the damaging effects of radiation. In the most recent study, the researchers used autoflourescence imaging to observe changes in cell metabolism after exposure to radiation and the chemotherapy drug YC-1. YC-1 has shown promise improving the effectiveness of radiotherapy on radiation-resistant cancer cells by inhibiting hypoxia-inducible factor.
The researchers observed significant metabolic differences between radiation-resistant and radiation-responsive cells following exposure to radiation only and exposure to YC-1 only. However, all differences between the resistant and responsive cells were eliminated when the researchers combined radiation and chemotherapy. These results suggest that YC-1 made radiation-resistant cells more sensitive to radiation treatment, and that this effect could be observed using autoflourescence imaging.
The large interdisciplary team led by Rajaram, includes Kyle Quinn, assistant professor of biomedical engineering; Kinan Alhallak, graduate student in biomedical engineering; Nicholas Greene, assistant professor of exercise science; David Lee, doctoral student in kinesiology; and Ruud Dings and Robert Griffin, faculty in the Department of Radiation Oncology at the University of Arkansas for Medical Sciences.
Funding for this work was provided by the Arkansas Biosciences Insititute, the National Institutes of Health and the National Institute of General Medical Sciences.
Previous research by this team compared radiation-resistant cancer cells to raditation-sensitive cells and found that radiation-resistant cells have higher levels of hypoxia-inducible factor, which regulates the amount of oxygen in the cellular environment and protects the cancer cells from the damaging effects of radiation. In the most recent study, the researchers used autoflourescence imaging to observe changes in cell metabolism after exposure to radiation and the chemotherapy drug YC-1. YC-1 has shown promise improving the effectiveness of radiotherapy on radiation-resistant cancer cells by inhibiting hypoxia-inducible factor.
The researchers observed significant metabolic differences between radiation-resistant and radiation-responsive cells following exposure to radiation only and exposure to YC-1 only. However, all differences between the resistant and responsive cells were eliminated when the researchers combined radiation and chemotherapy. These results suggest that YC-1 made radiation-resistant cells more sensitive to radiation treatment, and that this effect could be observed using autoflourescence imaging.
The large interdisciplary team led by Rajaram, includes Kyle Quinn, assistant professor of biomedical engineering; Kinan Alhallak, graduate student in biomedical engineering; Nicholas Greene, assistant professor of exercise science; David Lee, doctoral student in kinesiology; and Ruud Dings and Robert Griffin, faculty in the Department of Radiation Oncology at the University of Arkansas for Medical Sciences.
Funding for this work was provided by the Arkansas Biosciences Insititute, the National Institutes of Health and the National Institute of General Medical Sciences.