Affairs of the Heart

A case in point: Jensen himself has a leaking pulmonary valve, discovered by a colleague who assured him that 60 percent of adults suffer from the same condition. Which invites the question: if more than half of all adults have a leaky valve, is it really a defect or is it supposed to be like that?
Ultimately, Jensen says, “Many things can go wrong in the development process of the heart, and it’s one of the imperfect wonders of nature that creates something that is so remarkable. It’s a design that took 5 billion years to develop.”
To talk to Morten Jensen is to be immersed in the mystery and wonder of the human heart. Its leaky valves. Its coronary bifurcations and blockages. Its left ventricle muscles creating five Newtons of force every time they open and close the mitral valve (which he simulates by grabbing two 500ml plastic bottles and banging them down).
Oh, and the word mitral? It means “shaped like a mitre” in Latin, which is the pointed hat the pope wears.
When Jensen says of the heart, “it’s simple, beautiful, but incredibly complex,” it’s clear his fascination stems from a deep understanding of its function and mechanics.
Jensen came to the university in 2015 as an Arkansas Research Alliance Scholar and is now the principal investigator at the Cardiovascular Biomechanics Laboratory. Unfortunately, he has his work cut out for him: cardiovascular disease is the number one killer worldwide while Arkansas and its surrounding states have the highest death rates from cardiovascular disease in the United States. (the others are nearby Oklahoma, Mississippi, Louisiana and Alabama).
The CBLab brings engineers and clinicians together to address conditions such as heart attack, stroke, heart failure and congenital heart disease while creating novel approaches in the prevention, diagnosis and treatment of cardiovascular disease.
A Transformative Internship
Jensen got into the field almost by accident. When he was a computer and electrical engineering student in Denmark, where he was born, he was required to do a one semester internship on a research or industry-related project. He did this with the bioengineering department of Aarhus University Hospital, where he witnessed heart surgeries on humans, including a heart transplant. The experience set him on an entirely new course.
“I thought it was extremely fascinating with the heart being a pump,” Jensen explained. “You could look at the heart as a mechanical device, adding pressure to fluids and the fluids flowing into the blood vessels.”
He also witnessed researchers doing experiments on large animals.
“I saw what the researchers were able to do on large animals in the basement of that hospital — making measurements on the hearts, both electrical and force measurements, flow measurements and pressure measurements. And that’s why I became interested in this work.”
One of the people working in the basement was J. Michael Hasenkam, who was part of the team that invented the Transcatheter Aortic Valve Implantation, or TAVI, a revolutionary procedure that enables surgeons to implant a new heart valve with a catheter by going through a blood vessel in the armpit or groin. Ten years later, after Jensen had earned a master’s degree in biomedical engineering at the Georgia Institute of Technology, Hasenkam would become one of Jensen’s Ph.D. advisors back at Aarhus.
In 2015, Jensen became just the third engineer in Denmark since 1479 to obtain the prestigious Doctor of Medical Science degree, which is officially a higher doctorate, ranking above a Ph.D.
Since he saw that first heart transplant, Jensen has helped contribute intellectual property to nine patent applications. This has included development of lifelike artificial tissues to aid medical students in their training, spoons to assist with the removal of bladder stones on companion animals, and a heart valve created from animal tissues that can be implanted in humans.

Kaitlyn Elmer
Kaitlyn Elmer, a Ph.D. student in biomedical engineering from Springfield, Missouri, conducts research in Jensen’s lab. Elmer and Jensen are specifically focused on arterial blockages at a coronary bifurcation. This is where a major artery splits into smaller blood vessels. Elmer noted that “20 percent of plaque blockages are actually in a bifurcation. And since it’s branching, it’s just a weird shape and really difficult for physicians to use conventional methods to treat those.”
She said that normal methods employ a balloon to expand a stent that props the vessel open. These constructs are usually cylindrically shaped, but the bifurcation typically is not. So, she and Jensen are working with cardiologists to create a stent that is designed to fit in the bifurcation, which can have a range of angles. This has required development of a computer program to measure the angles of these bifurcations, so that they can create better designed balloons and stents. Prototypes are then created and tested in ballistic gel models to see how the stent’s shape changes when expanded.
The project is funded by the American Heart Association, and the technology is promising enough that they applied for two patents through the University of Arkansas’ Technology Ventures: one for the software program that measures the angle of bifurcation and another for the actual devices.
Vascugenix, a company based in Little Rock specializing in medical devices for interventional cardiology surgery, has signed an option agreement with Tech Ventures that would pave the way for licensing pending further development.
Noah Ascher, CEO of Vascugenix, said, “There is a clear need for a solution in the treatment of bifurcation lesions, and it is our company’s vision to work with Dr. Jensen to develop, manufacture and deliver a comprehensive stent system for addressing this need.”
Elmer confirmed that “Morten is really good at the industry and clinical partnerships. So I’ve been able to work with a clinician who has a lot of experience, Dr. [Barry] Uretsky. And Morten’s helped coordinate things with Vascugenix. So, I’ve learned a lot about the industry and business side of things.”
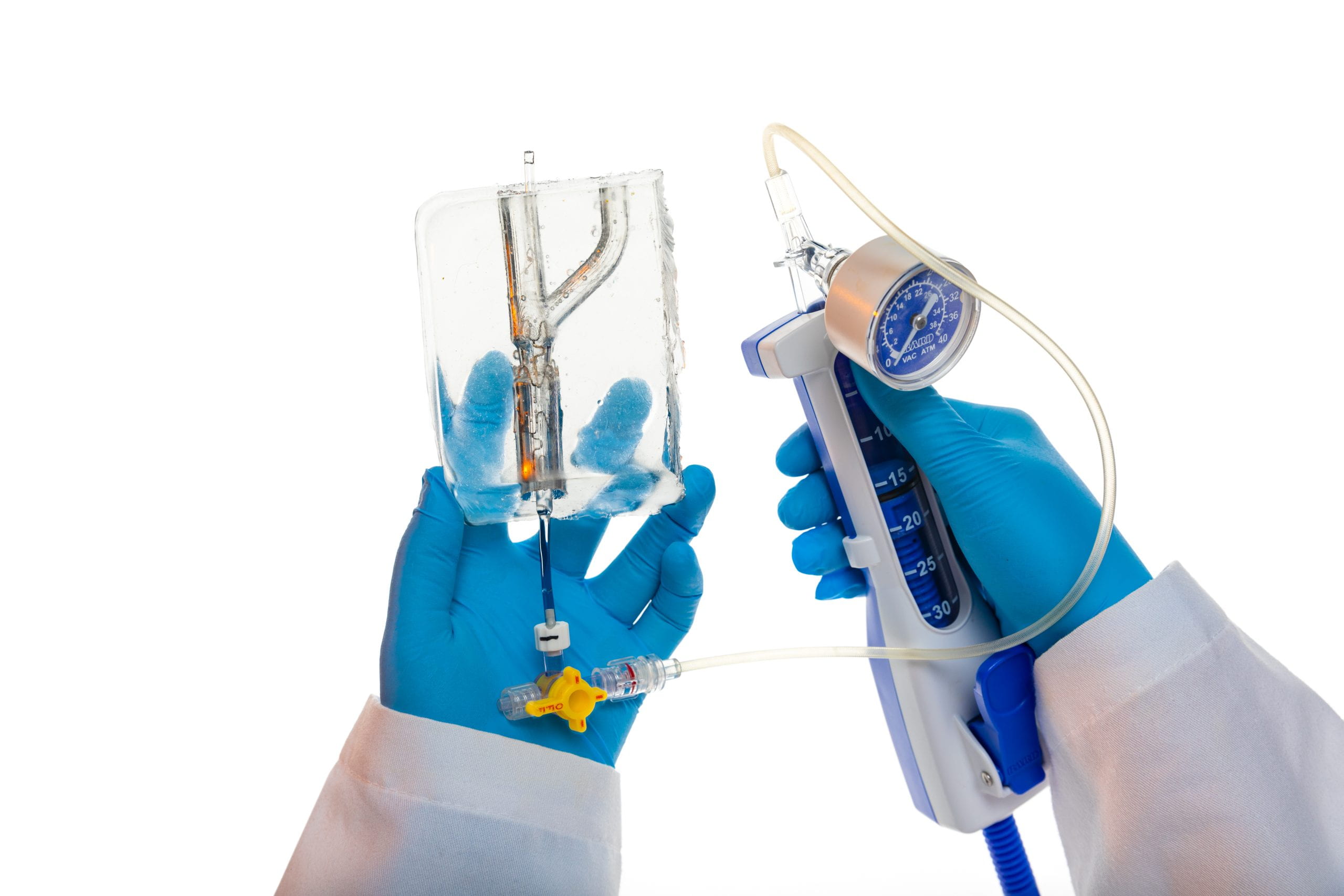
Jensen is also the principal investigator on a recent $1.9 million award from the Department of Defense to develop a wearable device for the early detection and monitoring of internal or external bleeding.

Jingxian Wu
Hemorrhagic shock is currently the leading cause of preventable death in casualty care settings. Existing methods often fail to detect blood loss until the onset of shock, which can be too late for some patients. This makes early detection and management of bleeding-related conditions critical to improving survival rates.
Jensen is working with a multi-disciplinary team to design a mobile device that can detect blood pressure waveforms, which correlate with the volume of blood within the blood vessels, the “intravascular volume,” and can be used to determine if blood volume is falling due to hemorrhaging. This will enable first responders and hospital staff to get more accurate readings earlier and respond with better timed and more precisely calibrated care.
Jensen will be joined by Jingxian Wu, a U of A professor of electrical engineering, and Robert Saunders, an associate department head of electrical engineering and computer science. Hanna Jensen, an assistant professor in the Department of Surgery at the University of Arkansas for Medical Sciences and course director of the school’s cardiovascular module, will oversee the translational and clinical phases of the project.
Ultimately, their goal is to develop a device that is less than an inch square and sells for less than $100. It would have a catheter that connects to a vein as well as a port to which an IV bag could be connected.
Collaboration Is Key

Robert Saunders
Saunders, who teaches a senior design class in the College of Engineering, has worked with Jensen on a range of projects. How many? “Wow, that’s a long list,” Saunders laughs before rattling off a few: “Heated pulse oximeters for people who have bad circulation. Wheelchair monitors for disabled people. An Ohm device to determine whether or not an artificial knee has appropriate motion.”
Saunders said of Jensen, “He’s a great part of the team. Everybody is really dedicated to the project they’re working on. They’re dedicated to the students around them.”
Collaboration is clearly key to Jensen’s success, from witnessing those teams in the Aarhus basement, to all the adjoining names on his patent applications, to his recent research projects.
In fact, one of his closest collaborators is his wife, Hanna, who is an M.D. She said she knew Jensen professionally before knowing him personally. Their research topics were so closely related that they kept presenting at the same sessions of the same conferences: her on the medical side, him on the engineering.

Hanna Jensen
“I distinctly remember Morten walking up to me after one of my first presentations and telling me he could write a program to automatically count the number of activated leukocytes on an intravital microscope video clip — something I had spent painstaking months doing manually alone in a dark room as a medical student,” Hanna Jensen said. “I always joke that it was the moment I knew I’d marry him!”
They’ve since collaborated on a number of projects, including the current DOD grant, a vector flow imaging study on pediatric patients to create detailed images of the internal structure and blood flow of babies’ hearts, and two children, Matilda and Lukas.
“We figured out we can constantly learn from each other and fill gaps on the continuum of biomedical research,” Hanna explained, “and we respect each other’s areas of expertise, so we rarely clash on professional topics. We also have distinct roles that work well for us in professional and personal life. Morten is the idea generator, and I am the organizer. We both love to be connected and plugged into our professional communities, and it’s been so rewarding to find collaborators on both the medicine and engineering side — we are both at our best as team players. Plus, when a big deadline is looming, we can gear the entire family schedule towards making it happen.”
Coda
In a recent conversation, Jensen offered a quick tour of the heart, showing how blood comes in from the lungs through the left atrium, passes through the mitral valve, which prevents it from flowing backwards, then enters the left ventricle and pushes on to the aorta. From there it circulates through the body until it finally returns to the heart through the right ventricle and is sent back to the lungs for more oxygen.
He concludes: “So it’s pretty neat, the way it works.”
He’s just a got a few more things to fix.




You must be logged in to post a comment.